Immuno Oncology Checkpoint Proteins

Immuno oncology (I-O) is the one of the most exciting research areas in biomedical science. Since the early discovery of CTLA4/CD80 and PD1/PDL1 interactions, scientists continue to discover novel ligands involve in T-cell activity modulations. The research results have been quickly translated in bedside benefit as a growing list of immune blockade drugs gaining FDA approval and perform miracles in cancer patients.
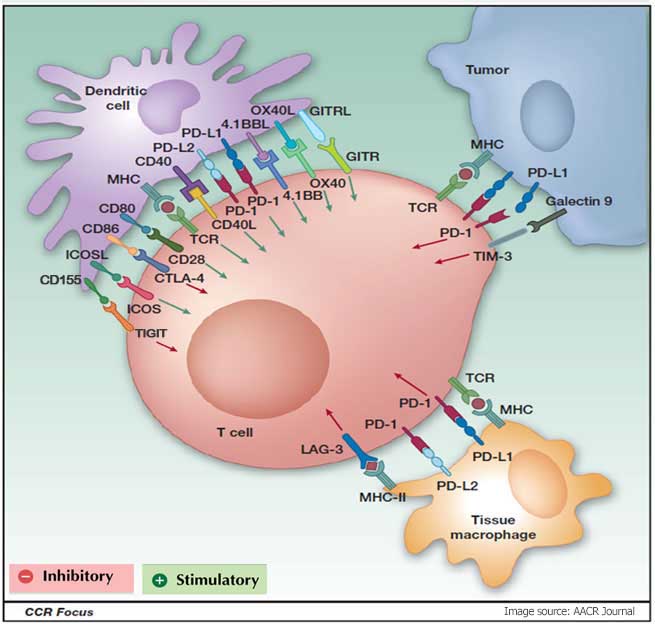
One of the most promising approaches is to activate the therapeutic anti-tumor immunity through the blockade of immune checkpoints. Immune checkpoints refer to a plethora of inhibitory pathways hardwired into the immune system that are crucial for maintaining self-tolerance and modulating the duration and amplitude of physiological immune responses in peripheral tissues in order to minimize collateral tissue damage.
It is now clear that tumors co-opt certain immune-checkpoint pathways as a major mechanism of immune resistance, particularly against T cells that are specific for tumor antigens. Because many of the immune checkpoints are initiated by ligand–receptor interactions, they can be readily blocked by antibodies or modulated by recombinant forms of ligands or receptors.
Cytotoxic T lymphocyte-associated antigen 4 (CTLA4) antibodies were the first of this class of immunotherapeutics to achieve US Food and Drug Administration (FDA) approval. Preliminary clinical findings with blockers of additional immune-checkpoint proteins, such as programmed cell death protein 1 (PD1), indicate broad and diverse therapeutic opportunities.
Listen to GENcast: Cancer Diagnostics and PD-L1

OriGene offers a complete set of research tools for I-O research.
Click to view a complete product lists for that target.
| Cellular responses | T cell/Effector cells | Antigen presenting cell /Tumor cells |
|---|---|---|
| Co-inhibitory | PD-1/PDCD1/CD279 | PD-L1/CD274 or PD-L2/PDCD1LG2 |
| CTLA-4/CD152 | CD80/B7/B7-1 or CD86 | |
| TIM-3/HAVCR2 | Galectin-9/GAL9/LGALS9 | |
| TIGIT | CD155/PVR | |
| LAG3 | MHC-Class II | |
| VISTA/C10orf54 | Unknown | |
| Unknown | B7-H3/CD276 | |
| Unknown | B7-H4/VTCN1 | |
| BTLA/CD272 | HVEM/TR2/TNFRSF14 | |
| A2AR | Adenosine | |
| KIR | MHC-Class I | |
| Co-stimulatory | CD28 | CD80/B7/B7-1 or CD86 |
| ICOS/CD278 | CD275/ICOSLG /B7RP1 | |
| CD40L/CD154 | CD40 | |
| CD137/4-1BB | CD137L | |
| CD27 | CD70/CD27L | |
| OX40/CD134/TNFRSF4 | OX40L/TNFSF4 | |
| GITR | GITRL | |
| SIRPα | CD47 |
Currently Approved Immune Checkpoint Inhibitors
| FDA approval date | Target | Product name | Drug name | Class | Company | The current indications |
|---|---|---|---|---|---|---|
| 2011, Mar 25 | CTLA-4 | Yervoy | Ipilimumab | IgG1, human | BMS | unresectable or metastatic melanoma |
| 2014, Sep 4 | PD-1 | Keytruda | Pembrolizumab | IgG4, humanized | Merck | metastatic melanoma; non-small cell lung cancer;head and neck cancer |
| 2014, Dec 22 | PD-1 | Opdivo | Nivolumab | IgG4, human | BMS | metastatic melanoma; non-small cell lung cancer; head and neck cancer |
| 2016, May 18 | PD-L1 | Tecentriq | Atezolizumab | IgG1, humanized | Roche | lung cancer; urothelial cancer |
References
- "Novel cancer antigens for personalized immunotherapies: latest evidence and clinical potential.." Wurz GT et al. Ther Adv Med Oncol. Jan 2016.
- "Immune Checkpoint Inhibitors: New Insights and Current Place in Cancer Therapy.." La-Beck NM et al. Pharmacotherapy. 2015 Oct.
- "Immune Checkpoint Blockade in Cancer Therapy.." Postow MA et al. J Clin Oncol. 2015 Jun.
- "The blockade of immune checkpoints in cancer immunotherapy.." Drew M. Pardoll. et al. Nature. 2012 Apr.
- "Aberrant PD-L1 expression through 3'-UTR disruption in multiple cancers.." Kataoka K. et al. Nature. 2016 May.






























































































































































































































































 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China